MDA-MB-157 Cell Line Derived Xenograft
MDA-MB-157 cells have been widely used as a model system for studying various aspects of breast cancer biology, including the molecular mechanisms underlying tumor development and progression, and the identification of potential therapeutic targets for the treatment of breast cancer.
The utilization of human cells in xenograft tumor models, like the MDA-MB-157 cell lines derived xenograft model (CDX), is imperative in therapeutic development. Breast cancer xenograft models enable investigation of human breast cancer cell therapeutics targeting migration, metastasis, apoptosis, anti-angiogenesis and anti-tumor growth in a human-similar tumor environment. Therapeutic studies in a cell culture setting cannot mimic tumor stroma, vasculature and cell-to-cell interactions as seen in xenograft models.
| MDA-MB-157 | ER–, PR–, HER2–, p53(mut) |
| Origin | Breast |
| Disease | Medullary Carcinoma |
| Metastatic Models (Breast) | 4T1 |
| Non-Metastatic Models (Breast) | BT-474, HS578T, KPL-4, MCF-7, MDA-MB-157, MDA-MB-231, MDA-MB-453, MDA-MB-468, T-47D |
Get Instant Quote for
MDA-MB-157 Xenograft Model
The preparation of tumor xenograft models requires cells growth of large number of tumor cells for inoculation. One million cells usually required for a single injection. Cell population is expanded and obtained by trypsinization from 225 cm2 tissue culture flasks. The collected cells are centrifuged and separated from the supernatant, followed by cell count (via flow cytometry) prior to matrigel injection.
MDA-MB-157 Basic Tumor Xenograft Study Design
- NOD/SCID mice (8-12 weeks old)
- Cells are in exponential growth prior to injection. Cell suspensions with greater than 99% viability are used.
- Each mouse receive one subcutaneous injection in the flank of the hind leg.
- Each site will receive one million tumorigenic cells in a volume of 100 uL containing 50% matrigel (use of matrigel matrix improves tumor cell survival and tumor formation).
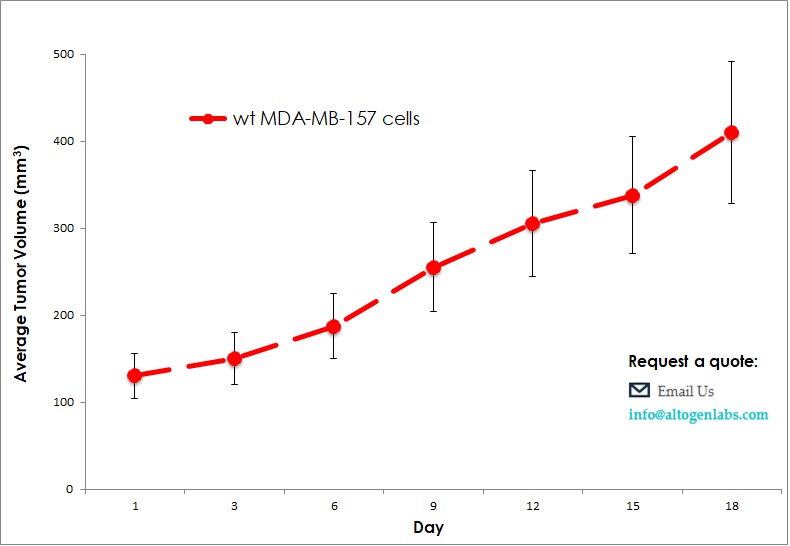
- The injection sites are palpated three times weekly until tumors are established. Tumors are measured using digital calipers until they reach an average size of 150-250 mm3. Injected animals are monitored daily with cage-side observations, and body weight measurements.
- Animals are randomized into treatment cohorts and treated according to the treatment schedule. We recommend including cyclophosphamide or other positive control treatment group.
- Once palpable tumors are observed, tumors are measured and mouse weights recorded 2 times a week.
- Gross necropsy and tissue collection performed as defined for termination of experiment. Animals showing signs of morbidity due to tumor burden or treatment toxicity are euthanized and processed as defined for termination of experiment.
- Tumors are excised, weighed and documented by digital imaging. Standard necropsies are performed and tissues collected for downstream processing and analysis.