Altogen Labs offers ‘human-in-mouse’ xenograft testing (patient derived xenografts, PDX) services for personalized medicine applications and drug development of anticancer therapeutics. Science article about our PDX methodology, if you are a cancer patient interested in ordering PDX xenograft and chemosensitivity testing services, please visit our patient testing webpage
The use of preclinical models for drug testing is central to translational cancer therapeutics. Although mouse CDX xenograft models (cell line derived xenograft) derived from mono-cellular layers of tumor cells cultivated in vitro serve as useful tools, they often fail to recapitulate the key aspects of human malignancies and thus do not accurately predict drug effects in the clinic. Studies show mouse xenografts that have been selected and properly characterized indeed have utility for predicting responsiveness to targeted agents, however, they do not replicate the tumor micro-environment and interactions between the tumor and the innate immune system.
Tumor graft models, i.e. Patient Derived Xenografts (PDX), are created by implanting primary human tumor material directly into an immunodeficient host (laboratory rats and mice). The host being used must be immunocompromised to prevent transplant rejection. Several types of immuno-deficient hosts are used to establish PDX models like nude or NOD-SCID mice. The NOD-SCID mouse is considered more immunodeficient than the nude mouse as they lack NK cells (natural killer cells), and therefore is more commonly used for PDX models. These models have an advantage over CDX models as the tumor retains a more natural architecture and are more reflective of the heterogeneity and histology seen in primary tumors.
| Number | Characteristics | Cell Line in vitro | Cell Line Xenograft | PDX | Patient |
| 1. | Heterogeneity | Nil | Limited | Higher intra tumor heterogeneity | High intra tumor heterogeneity |
| 2. | Molecular sub types | Modest diversity | Modest diversity | Diverse range of molecular subtypes | Full range of molecular subtypes |
| 3. | Stroma | Nil | Murine stroma/ No human stroma | Mixed murine & human stroma | Human stroma |
| 4. | Growth Rate | Rapid | Rapid | Slow | Chronic |
| 5. | Stages | Mixed primary and metastatic | Mixed primary and metastatic | Mixed primary and metastatic | Predominately metastatic |
| 6. | Immune system | Nil | Limited | Severely Limited | Fully active |
| 7. | Clinical outcomes | Nil | Nil | Limited | Available |
Table 1. Comparison of the PDX with previously established cell-lines, cell line xenograft and donor tumor from which they are derived.
PDX method and application
The tumor fragments harvested from the patient can be transplanted into the flanks or into particular organs of the immunocompromised mice that are maintained under controlled laboratory conditions and carefully monitored (Miles et al, 2014). The immune deficiencies of the laboratory mice enhance the formation of xenografts that have the unique features of the engrafted tumor and allow the optimization of the specific therapy. The xenografts derived from human tumours can also be analysed in order to identify their special characters, the mutations that affect them and the influence of certain factors on the gene expression.
The mice with established tumours are identified after monitoring the entire group and used as models for developing new therapies and for studying the progression of metastasis, especially useful for patients with rare cancers (Siolas and Hannon, 2013). The patient derived xenografts retain the gene expression profile of the engrafted tumour and their response to the treatment is similar to the response obtained during the clinical trials (Siolas and Hannon, 2013).
Examples of xenograft personalized medicine applications
Lee e al. studied triple negative breast cancer xenografts for the inhibition of cancer growth and metastasis by a biomimetic peptide derived from collagen, namely SP2043, which can be encapsulated in order to ensure its stability in vivo (2014).
The Notch ligand δ-like 4 is involved in the cancer angiogenesis and influences the tumour growth. The research performed on a RP-R-01 patient derived xenograft provided very useful information regarding the effect of various anti-Dll4 drug combinations on cancer development (Miles et al, 2014). The results of the study can be very useful in the development of a new therapy against renal cancer.
Navarro et al. described the positive role of the natural killer cells against the glioblastomas, which are therapy resistant (2014). The NK cells are involved in the mediation of the cancer cells lysis, and the results of the research encourage the scientists to continue the tests for developing an effective treatment against chemotherapy resistant cancers.
Einarsdottir et al. recommend the use of patient derived xenografts for studying the particular malignant melanomas and for choosing the best treatment for the donor (2014). The researchers performed a detailed characterization of the obtained xenografts, proving the similarities between them and the human melanomas (Einarsdottir et al, 2014).
The role of let-7d miRNA in the progression of the renal carcinoma was studied in vivo on patient-derived xenografts, suggesting the fact that these molecules are capable of inhibiting the renal cell carcinoma growth and metastasis because of their influence on the evolution of the stromal cells (Su et al, 2014). The authors recommend further studies for the development of a new therapeutic approach for the renal cell carcinoma metastasis.
Leong et al. generated patient-derived xenografts by implanting the small cell lung cancer samples obtained through endobronchial ultrasound-guided transbronchial needle aspiration into the flanks of nude mice (2014). The new method can be applied for easily obtaining the models for testing new drugs or for performing genomic analysis.
The effect of chemotherapy and radiotherapy in treating the squamous cell carcinomas of head and neck is enhanced by the inhibitory effect of statins on the epidermal growth factor receptor (de Llobet et al, 2014). The authors described the positive role of simvastatin in wound healing, cells survival, and the inhibition of tumour growth during radiotherapy and chemotherapy.
Gökmen-Polar et al. described the results of their research regarding the pathways of the resistance of breast tumours against bevacizumab, a compound that enhances cancer progression and metastasis (2014). The analysis of the gene expression provided useful information regarding their regulation during the treatment.
Patient-derived xenografts were used by Annibali et al. for studying the inhibitory effect of Myc genes on the development of glioma by decreasing the division rate of the cancer cells (2014). The authors obtained orthotropic xenografts by implanting the human tumour cells into the mice corpus striatum and proved the inhibitory effect of the Myc genes on mitosis.
The patient derived xenografts are very useful tools for performing preclinical tests of new cancer therapies, for gathering useful information regarding rare cancers and for studying the influence of many factors on the cancer progression and metastasis. The studies on these tumors can provide new ideas for starting new research paths or for finding new approaches for old therapies.
PDX methodology
To establish a PDX model, patient tumors are obtained fresh from surgery, mechanically sectioned in to fragments via chemical digestion or physically manipulated in to single-cell suspensions. They are then injected into NOD-SCID mice. Use of tumor fragments retains cell-cell interactions and mimics the microenvironment while single cell suspensions enable unbiased sampling of whole tumor by eliminating segregates of sub clones. Cell viability and engraftment success is lower in the single cell method compared to the tumor fragment method.
Tumors can be injected heterotopically or orthotopically. Heterotopic models involve injecting tumors in to the subcutaneous flank of a host. This provides easier cell transfer and precise monitoring of the growth and location. An orthotopic model involves direct implanting of tumor on an organ of choice. It is more technically challenging and time consuming. In some cases in vivo imaging is required to verify tumor grafts after implantation, but they accurately mimic the human tumors from which they are derived in the manners of histology and gene expression. The generation harboring the patient-derived material is termed F0, with subsequent generations numbered consecutively (F1, F2, F3 and so on). It takes roughly 2 to 3 months for the tumor to engraft, depending on the tumor type, implant location and mice strain utilized. The engraftment failure should not be decided until at least 4-6 months have passed. The model can be validated by comparing histopathologic, biologic and genetic features to the donor’s tumor. Studies have shown that these models retain donor tumor characteristics and these traits are maintained through successive mouse generations.
For drug development studies, expansion of mice after the F3 generation is often utilized. Multilayered biological assays like drug efficacy studies, combination studies and development of predictive biomarkers for novel therapies are performed on early generations (F3:F5), after ensuring that the PDX has not genetically or histologically diverged from the patient’s tumor.
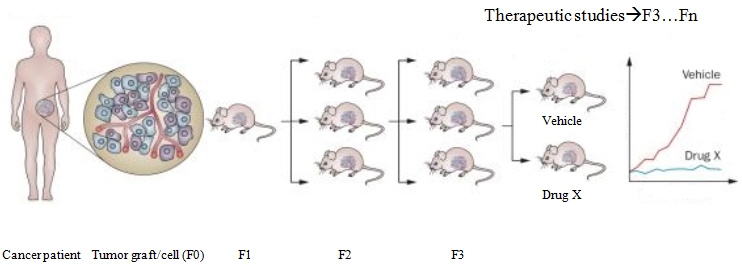
Figure 1. Establishment and testing of PDX models.
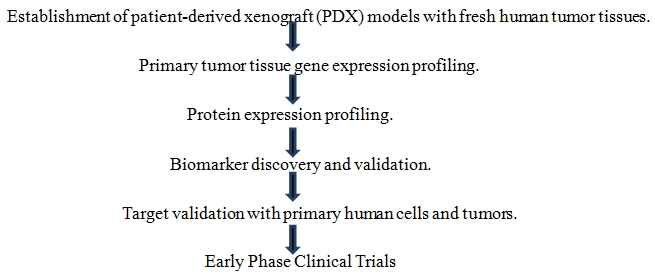 Figure 2. Steps involved in standard oncology drug studies
Figure 2. Steps involved in standard oncology drug studies
PDX models are capable of maintaining the human malignancy complexity remarkably well. Hence, they have extraordinary utility in basic research and have demonstrated predictive power in novel drug efficacy studies, biomarker analysis and patient outcome.
Example of the PDX protocol:
Primary tumor processing and heterotopic implantation:
- Sterilize bioguard safety hood with application of copious amounts of 70% ethanol. Sterilize all surgical instruments in an autoclave and allow cooling to room temperature.
- Transfer tumor specimen into a sterile 50 mL centrifuge tube containing 30 mLs of sterile, serum-free RPMI supplemented with 1x antibiotic/antimycotic (antibiotic media) chilled to 4°C.
- All surgical specimens must be assumed to be bio-hazardous and handled with extreme caution using universal precautions. Acquire excess tumor in accordance with institutional review board guidelines and regulations only after adequate tissue diagnosis and evaluation of tumor margins have been performed by a staff pathologist.
- Time interval between tumor resection to implantation in mice must be kept to a minimum. Ideally, this should occur in less than one hour after resection with tumor maintained in serum-free antibiotic media, on ice.
- Wash tumor sample twice with 30 ml of serum-free antibiotic media chilled to 4°C to reduce contaminant load.
- Weigh NOD/SCID mice and anesthetize using IACUC approved method (e.g. ketamine/xylazine cocktail at ratio of 100 mg/kg: 20 mg/kg).
- All animal experiments require approval by institutional review board and animal use and care committees (IACUC) and must be conducted in accordance with institutional and national regulations.
- Once anesthetized, shave left abdominal/flank region of NOD/SCID mouse with electric clippers and place mouse on its right side. Paint left side of mouse from base of neck to tail with gauze soaked in 70% ethanol solution.
- In a sterile, ventilated hood, place tumor specimen on a sterile petri dish and cover with antibiotic media. Mince tumor with a sterile #21 blade until 1–2 mm3 tumor fragments are obtained. Do not allow tumor to dry; keep immersed in antibiotic media at all times.
- Incise skin with microscissors and implant tumor fragments into subcutaneous space. Incision should involve only skin layers and not deep muscle layers. The skin is highly mobile and identified when grasped and lifted from deep tissue by forceps.
- While holding incision site open with forceps, gently lay five 1–2 mm3 tumor fragments onto underlying tissue. Tumor fragments should individually remain adherent to underlying tissue within formed subcutaneous tunnel.
- Close skin incision with interrupted stitches (3-0 silk suture). Close skin with interrupted 3-0 silk sutures.
- Recover mice from the effects of anesthetic.
Heterotopic passage of xenograft tumor:
- Mice should be sacrificed and tumor harvested once tumor sizes reaches 1.2–1.5 cm in largest diameter or upon ulceration of skin.
- Weigh tumor-bearing and recipient NOD/SCID mice and anesthetize using IACUC approved method (e.g. ketamine/xylazine, 100 mg/kg: 20 mg/kg).
- Once anesthetized, shave tumor-bearing region of mice with electric clippers and repeat at desired site of tumor implantation in recipient NOD/SCID mice. Paint tumor region or recipient site of mice from base of neck to tail with gauze soaked in 70% ethanol solution.
- Dissect xenograft tumor from adherent subcutaneous tissue using sterile forceps and sterile, curved microscissors. Start by incising skin at the base of the xenograft tumor. Insert miroscissor tips into incision and gently spread, slowly developing a dissection plane between skin and underlying tumor. Extend incision as needed to completely uncover tumor and dissect the tumor from underlying deep tissue.
- Once tumor is free, place in sterile, serum-free RPMI supplemented with 1x antibiotic/antifungal (antibiotic media) chilled to 4°C. Wash specimen twice with 30 mL volumes of serum-free antibiotic media.
- Mince tumor with a sterile #21 blade until 1 mm3 fragments are obtained and proceed as described above for the implantation of original patient tumors into recipient NOD/SCID mice.
- Recover mice from the effects of anesthetic.
Processing of xenograft tumor into a single cell suspension
- Prepare collagenase IV solution (200 units/mL) and filter sterilize.
- Euthanize mouse in CO2 chamber and shave tumor site with electric clippers. Paint tumor site with 70% ethanol and harvest tumor with sterile instruments in a sterile hood.
- Wash tumor twice with 30 mLs of serum-free antibiotic media. Place tumor on petri dish and cover with 2 mLs of collagenase IV solution.
- Do not allow tumor to dry; keep immersed in collagenase IV solution at all times.
- Using a sterile #21 blade scalpel, mince tumor into smallest possible fragments (1.0 mm3) and place 0.2 g of tumor (paste-like consistency) into each well of a 6-well plate. Place 5 mls of collagenase IV solution into each well and pipette with 25 ml pipette. Place 6-well plate(s) into 37°C incubator for 2–2.5 hours, mixing with a 10 ml pipette every 20 minutes to augment digestion. Continue to mince tumor fragments to smallest possible pieces prior to pipetting.
- After incubation, fill each well with 8 ml of sterile, serum-free RPMI and mix suspension with a 25 ml pipette.
- Strain digested tumor solution through sterile 70 µM cell strainer, collecting cell solution in a 50 ml centrifuge tube. Discard filter and associated tissue fragments.
- Centrifuge cell solution at 1200 rpm for 5 minutes and remove supernatant. Add RBC cell lysis buffer 1:10 volume pellet:buffer and re-suspend. Place on ice for 10 minutes.
- Centrifuge solution at 1200 rpm for 5 minutes and remove buffer. Re-suspend in serum-free RPMI and pass cell solution through 40 µm cell strainer. Count cells using flow cytometry system.
- Proceed to orthotopic or heterotopic injection into immunodeficient mice (see below), or antibody and/or dye staining for flow cytometry.
Anticipated results
Palpable growth of patient tumor in mice may take 4–10 weeks with an average time 6-8 weeks. However, once passaged into subsequent generations of mice, maximum time until tumor formation should be about 4-6 weeks. Orthotopic tumors derived from xenograft tumors may take 8–12 weeks to reach tumor sizes palpable through the abdominal wall. While it is possible to implant xenograft tumor at heterotopic and orthotopic sites in the same mouse, we discourage this practice if sizeable tumors are required for experimental purposes as animals will likely require euthanasia for heterotopic tumor size prior to the development of substantial tumor size. Tumors derived from established cell lines (heterotopic or orthotopic) form palpable tumors between 2–5 weeks post-implantation.
Read more about our PDX services
References:
Lewis, M. T., and Caldas, C. (2024). The Power and Promise of Patient-Derived Xenografts of Human Breast Cancer. Cold Spring Harbor perspectives in medicine, 14(4), a041329.
Siolas D., and Hannon GJ. (2013) Patient Derived Tumor Xenografts: transforming clinical samples into mouse models.
Janitri, V. et al. (2024). The roles of patient-derived xenograft models and artificial intelligence toward precision medicine. MedComm, 5(10), e745.
Kopetz S., Lemos R., and Powis G., (2012). The Promise of Patient-Derived Xenografts: The Best Laid Plans of Man and Mice. Clin Cancer Res, 18:5160-5162.
Tentler JJ., Tan AC., Weekes.CD., Jimeno A., Leong S., Pitts TM., Arcaroli JJ., Messersmith WA., and Eckhardt SG., (2012), Patient-derived tumour xenografts as models for oncology drug development, Nat Rev Clin Oncol. 9(6):338-350.
Morton Cl., and Houghton PJ., Establishment of human tumor xenograft in immunificient mice (2007). Nat Protocol; 2(2):247-50.
Hidalgo M., Amant F., Biankin AV., Budinska E., DeJong S., Jonkers J., Maelandsmo GM., Roman-Roman S., (2014), Patient-Derived Xenograft Models: An emerging platform for translational cancer research Cancer Discov; 4(9); 998–1013.
Aslani, S., and Saad, M. I. (2024). Patient-Derived Xenograft Models in Cancer Research: Methodology, Applications, and Future Prospects. Methods in molecular biology, 2806, 9–18.