PC-3 Cell Line Derived Xenograft
PC3 cells are a human prostate cancer cell line that was originally isolated from the bone marrow of a patient with advanced prostate cancer in 1979. PC-3 cells are commonly used as a model system for studying prostate cancer and evaluating potential therapies. PC3 cells are known to exhibit a variety of genetic alterations that are commonly found in prostate cancer, including mutations in the TP53 and PTEN genes. They also produce high levels of prostate-specific antigen (PSA), which is a marker for prostate cancer. Studies using the PC3 cell line have provided important insights into the biology of prostate cancer and have helped identify potential targets for new therapies. For example, PC3 cells have been used to study the role of androgen receptors in prostate cancer and to test new drugs that target these receptors. However, it is important to note that findings obtained using cancer cell lines, including PC3, should be confirmed using other models, such as patient-derived xenografts or genetically engineered mouse models, before being translated to clinical trials in humans. Preclinical experiments to determine efficacy of novel therapeutic compounds rely on the PC-3 CDX model (cell line derived xenograft). Xenograft models entail subcutaneous injections of human cancer cells into the rear flank of immunocompromised mice (e.g. Nude or NOD/SCID). Much like human tumors, researchers utilizing rodent prostate cancer models have to consider castration-resistance and androgen dependence (AD). There is a high demand to find a new diagnostic marker that replaces the dependence on prostate-specific antigen (PSA) to monitor progression. As well, there is an increasing need for novel therapeutic targets that decrease commonly monitored parameters such as to reduce VEGF expression and microvessel density, and maximize tumor growth inhibition (e.g. silibinin).
| Mutations | PTEN(del), p53(del) |
| Origin | Prostate |
| Disease | Adenocarcinoma, Grade IV |
| Metastatic Models (Prostate) | PC-3 |
| Non-Metastatic Models (Prostate) | DU145, LNCaP |
Metastatic Model
Proliferating tumor cells invade local tissue, travel through the circulatory system and implant in a foreign tissue. Metastasis leads to high death rates in cancer patients. Cell line derived xenograft (CDX) mouse models are highly utilized in preclinical assessment of novel cancer therapeutics and are a crucial link between initial high-throughput in vitro screening data and anti-tumor efficacy. Metastatic mouse models are utilized to understand the interactions of the anti-metastatic therapeutic and tumor in regards to all organs, bioavailability (e.g. half-life), clearance, immune response and tumor efficacy. Due to the inability to palpate metastatic tumors, the insertion of a luciferase (bioluminescence) or GFP (fluorescence) gene into the genome of the cell line of interest enables the researcher to visually track and quantitate internal tumor progression throughout the in-life portion of the animal study.
U87-Luc MG Xenograft Model (case study)
U-87 MG cells expressing luciferase were implanted and the tumors were allowed to grow. Using a Night Owl (Berthold Technologies), tumor growth was monitored throughout the study after an intraperitoneal (IP) injection of luciferin. An image of tumor location and the ability to capture a quantifiable value for orthotopic or metastatic tumor progression is the main strength of the luciferase expression (emitted photons) in the U87 MG-Luc model.
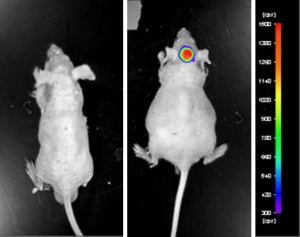
Figure: Expression of luciferase in U87-Luc MG orthotopic model. Control (left) and implanted glioma mouse model fluorescence (right) was captured after intraperitoneal luciferin injection (10 min incubation).
View details of the case study here.
Get Instant Quote for
PC-3 Xenograft Model
What is a Xenograft?
Development of an anti-cancer therapeutic requires intense, well planned studies that follow a streamlined path for success. Primary studies are performed in an in vitro setting that allows for high throughput screening and analysis of multiple compounds of interest. This method enables a focused compound screening approach of multiple cell lines within a specific cancer type, or a divergent approach across a broad range of cancer types. Ultimately, in vitro screening results need to be confirmed in an animal model due to in vitro inadequacies of cells cultured on plastic, as this method is far removed from the microenvironment of a tumor.
As the logical next step in therapeutic development is the administration of the test compound in a living animal, a cell line derived xenograft model (CDX) is created by inoculating human cancer cell lines in test animals. The injected cell lines grow into established tumors, thus, permitting efficacy studies of the test compounds. An alternative to CDX models is the patient derived tumor xenograft (PDX) which consists of implanting human tumor fragments directly in a mouse model. The PDX model avoids concerns with the CDX model since the tumor is never grown on plastic and there is no selection for single cell populations. In contrary to CDX models, the ideology of PDX models is to maintain the cell population, structure and stroma of the initial tumor.
Why use Xenograft Models?
Cell line derived xenograft (CDX) models or patient derived tumor xenograft (PDX) models enable a larger realm of parameters to be studied not capable with in vitro studies. The complete animal system model expands the scope of studies available to include the effect of test compounds on pharmacokinetics (PK), pharmacodynamics (PD), alternate routes of delivery, inhibition of metastasis, CBCs, dosing regimens, dose levels, etc. However, one of the major drawbacks of CDX and PDX models is that the human cancer cell lines or human patient derived tumors must be implanted in immunocompromised mice in order to bypass the graft versus host rejection by the animal. With the increasing focus of the immune systems role in the recognition and elimination of tumor cells (i.e. immunotherapy), major consideration must be taken into account during experimental concept design of the limitation of checkpoint inhibitors or desired immune response involvement in tumor efficacy. Similarly, any tumor regression after treatment with a test compound in these models will not exhibit the potential complement cascade or innate immune response of the injected therapeutic in humans.
What we offer?
Our in vivo xenograft service department evaluates the efficacy of preclinical and clinical cancer therapeutics utilizing more than 90 validated immunocompromised xenograft mouse models. The value of utilizing our xenograft service department is highlighted by the ability to completely characterize the efficacy, dose regimen, dose levels and optimal combination ratios of lead compounds for cancer, obesity, diabetes, infections and immunology research.
During the design and execution of the xenograft study, our scientists will communicate with and assist the client’s decisions regarding these details:
- Study Group Formation: classification of mice by body weight, tumor size or other parameters
- Cancer Cell Line: use of in-house cell lines or utilization of customer-provided cell lines
- Tumor Implantation: intraperitoneal, subcutaneous, submuscular or intravenous
- Test Compound Administration: intraperitoneal, intravenous, tail vein, subcutaneous, topical, oral gavage, osmotic pumps or subcutaneous drug pellets
- Sample Collection: Tumors/tissues can be fixed in 10% NBF, frozen in liquid N2 or stabilized in RNAlater; blood chemistry analysis can be performed throughout the in-life portion of study
Vivarium
Our vivarium is designed such that it enables cost-effective and first-rate preclinical effectiveness testing services. All animal handling and maintenance is regulated following IACUC guidelines. Our facility consists of the following:
- IACUC-regulated and GLP-compliant
- Controlled, limited access lab areas
- Disposable cages
- Sterile food and water
- SPF (specific pathogen-free) animals to guarantee pathogens do not interfere with the experiment
- Established animal handling and micro-injection equipment systems, including an animal health observation program
- All studies follow pre-approved SOPs
Our staff understands that each proposed study design is unique and customized to the client’s needs. We also recognize the importance of the delivered results as being confidential, highly reproducible and that 100% of the intellectual property (IP) is owned by the client.
In order to receive a quote for your xenograft study, email us the specific details listed below in order to efficiently begin the study quote process:
- Cancer cell line(s) used in the study
- Number (n=) of animals in each study group
- Number of study groups and control groups
- Tumor implantation route
- Administration route of test compound
- Species of immunocompromised mouse (e.g. NOD/SCID, athymic Nude)
- Treatment and dose schedule
- Study endpoint and analysis (e.g. tumor growth delay, PK/PD, survival, toxicity, drug combinations)
- Samples collected: tumor and tissues to be collected, including storage condition (e.g. snap frozen, RNAlater, 10% NBF, nucleic acid isolation)