U87-Luc MG Cell Line Derived Xenograft (Luciferase expressing U-87 cells)
U87Luc cells are a modified version of the U87 glioblastoma cell line that have been engineered to express a luciferase gene. Luciferase is an enzyme that catalyzes a reaction that produces light, and by introducing the luciferase gene into U87 cells, researchers can track the growth and spread of these cells in real-time using bioluminescence imaging. The U87-Luc MG CDX mouse model is used as a preclinical model to test novel anti-angiogenic therapies (e.g. zoptarelin doxorubicin). Stable expression of the luciferase gene in the U-87 MG cell line allows real-time quantitation of emitted photons during in-life tumor growth. Observing expression of luciferase enables quantitation of anti-tumor activity from monotherapies (e.g. COTI-2) or combination therapies (e.g. PI-103 and temozolomide, milciclib and temozolomide).
| U-87 MG | CDKN2A(del), PTEN(mut) |
| Origin | Brain |
| Disease | Glioblastoma, Astrocytoma |
| Metastatic Models (Brain) | U-87 MG, U87-Luc MG |
| Non-Metastatic Models (Brain) | LN-229, SF268, SF295, SF539, SK-N-AS, SNB-19, SNB-75, U251 MG |
Metastatic Model
Proliferating tumor cells invade local tissue, travel through the circulatory system and implant in a foreign tissue. Metastasis leads to high death rates in cancer patients. Cell line derived xenograft (CDX) mouse models are highly utilized in preclinical assessment of novel cancer therapeutics and are a crucial link between initial high-throughput in vitro screening data and anti-tumor efficacy. Metastatic mouse models are utilized to understand the interactions of the anti-metastatic therapeutic and tumor in regards to all organs, bioavailability (e.g. half-life), clearance, immune response and tumor efficacy. Due to the inability to palpate metastatic tumors, the insertion of a luciferase (bioluminescence) or GFP (fluorescence) gene into the genome of the cell line of interest enables the researcher to visually track and quantitate internal tumor progression throughout the in-life portion of the animal study.
U87-Luc MG Xenograft Model case study
U-87 MG cells expressing luciferase were implanted and the tumors were allowed to grow. Using a Night Owl (Berthold Technologies), tumor growth was monitored throughout the study after an intraperitoneal (IP) injection of luciferin. An image of tumor location and the ability to capture a quantifiable value for orthotopic or metastatic tumor progression is the main strength of the luciferase expression (emitted photons) in the U87 MG-Luc model.
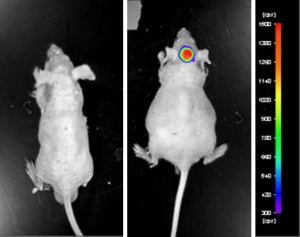
Figure: Expression of luciferase in U87-Luc MG orthotopic model. Control (left) and implanted glioma mouse model fluorescence (right) was captured after intraperitoneal luciferin injection (10 min incubation).
View details of the case study here.
Get Instant Quote for
U87-Luciferase Xenograft Model
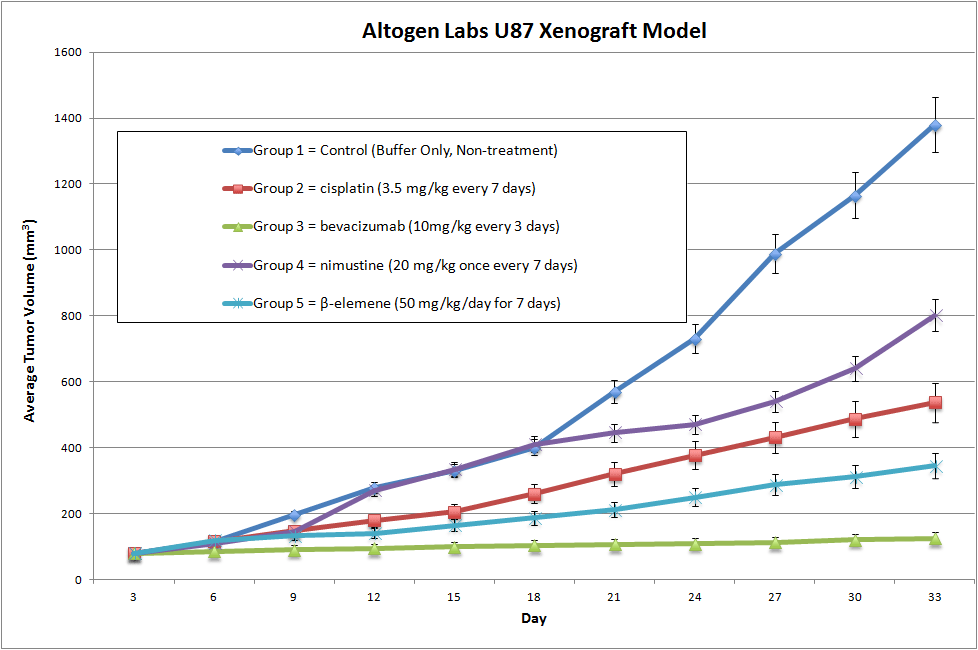
U87-Luc Basic Tumor Xenograft Study Design
NOD/SCID or Nude (Nu/Nu) mice (8-12 weeks old) are acclimated to the facility for 5-7 days prior to tumor cell injection/implantation.
Cells to be used for injection are maintained under conditions of exponential growth prior to injection. Cells are prepared for injection by trypsinization and viable cell counts are determined using trypan blue exclusion.
Cell count performed by flow cytometry and suspension adjusted to appropriate density. Only cell suspensions with greater than 98% viability are used.
Each mouse receive one subcutaneous injection in the flank of the hind leg.
Each site will receive one million tumorigenic cells in a volume of 100 uL containing 50% matrigel (use of matrigel matrix improves tumor cell survival and tumor formation).
The injection sites are palpated three times weekly until tumors are established. Tumors are measured using digital calipers until they reach an average size of 150-250 mm3. Injected animals are monitored daily with cage-side observations, and body weight measurements.
Animals are randomized into treatment cohorts and treated according to the treatment schedule. We recommend including cyclophosphamide or other positive control treatment group.
Once palpable tumors are observed, tumors are measured and mouse weights recorded 2 times a week.
Animals are euthanized when tumor size reaches 2,000 mm3 size limit. Gross necropsy and tissue collection performed as defined for termination of experiment. Animals showing signs of morbidity due to tumor burden or treatment toxicity are euthanized and processed as defined for termination of experiment.
Tumors are excised, weighed and documented by digital imaging. Standard necropsies are performed and tissues collected for downstream processing and analysis.